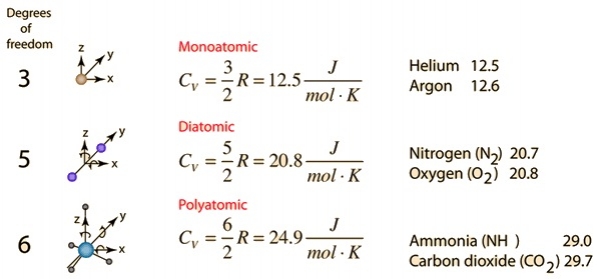
Constant Volume specific heat:-
Using 1st law of thermodynamics,
?U = Q – W
=Q , Where, W = P?V = Px0 = 0. Cos ?V = 0
?U = Cvn?T
=> Cv = ?U/n?T …………… (I)
Cv = Constant Volume Spacific hit.
For Monoatomic ideal gas:-
U = 3/2 nRT
?U = 3/2 nR?T ------------- (II)
From (I) and (II),
Cv = 1/n?T 3/2 nR?T
=> Cv = 3/2 R
R = Monoatomic Gas.
Constant Pressure specific heat:-
Cp = Cv + R
= 3/2 R + R
= 5/2 R
Proof:- Cp = Cv + R
The molecule specific heat at constant pressure is defined by,
Cp = Q/n?T
=> Q = Cpn?T ................... (I)
Using 1st law of thermodynamics
?U = Q - W
=> ?U + W = Q
=> ?U + P?V = Cpn?T , Where, W = P?V ............................... (II)
Form ideal gas law,
PV = nRT
=> P?V = nR?T ........................... (III)
From (II) and (III)
?U + nR?T = Cpn?T
=> ?U/?T + nR?T/?T = Cpn?T/?T [ Devided by '?T' ]
=> ?U/?T + nR = Cpn
=> Cp = 1/n ?U/?T + R
=> Cp = Cv + R (proved)